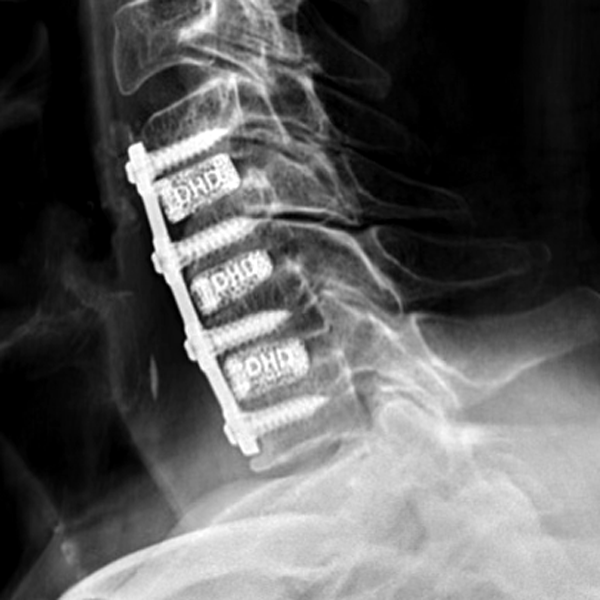
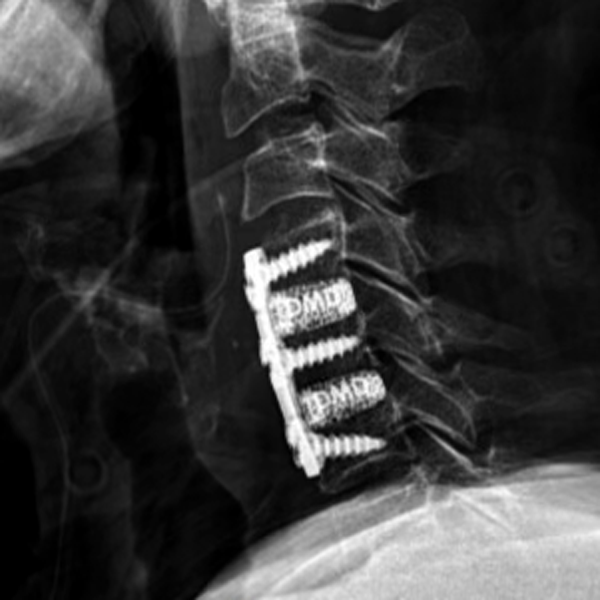
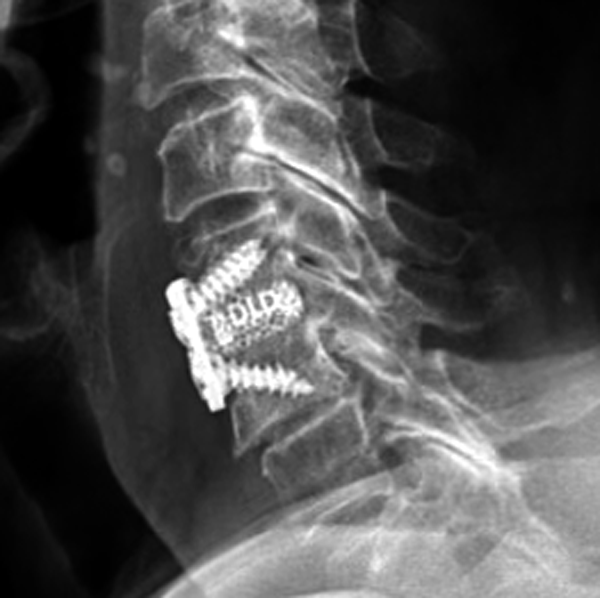
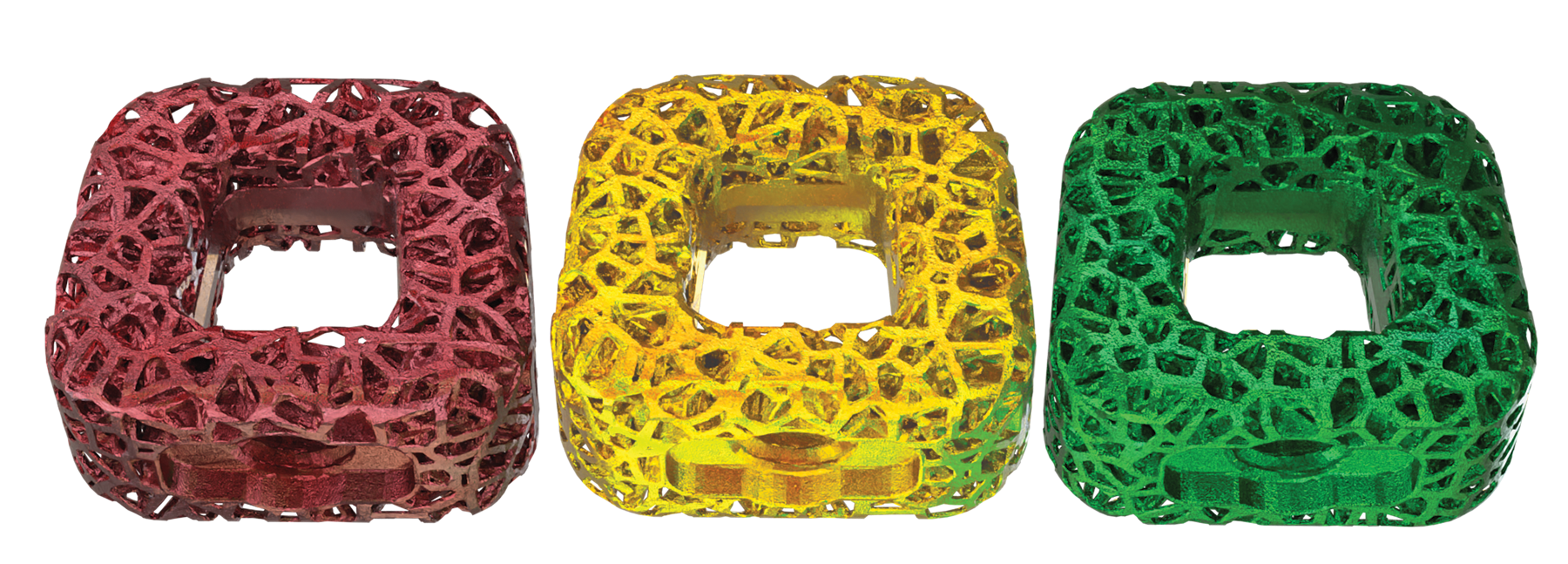Frameless cervical cages made of color-coded Titanium
“The DEXA-C™ implant from Aurora Spine ushers in a new era of personalized spine surgery. This customization prevents implant subsidence and accelerates fusion even in healthy individuals. This will expand access to spine surgery for older patients who were previously excluded by poor bone quality.”

Evolution of Cervical Spine Interbodies
Machined Metal
Too rigid
Subsidence issues
Not for osteoporotic bone
PEEK
Osteo-phobic
Non-union
Bone doesn’t attach
Machined Bone
Supply issues
Disease transmission
Weak structure
Bone Chips
No structure
Disease transmission
Inconsistent
3D Printed Cages
Too rigid
Subsidence issues
Endplate fractures
Ti-Coated PEEK
Osteo-phobic
Osteo-conductive top & bottom only
All the positive elements in one patented device
Function
Achieve stability while maintaining flexibility to match the patient’s bone density
Enhanced Stability
High coefficient of friction, for more solid initial fixation
Reduced risk of migration & expulsion
Excellent Flexibility
Stiffnesses similar to normal, osteopenic, and osteoporotic endplates
Provides for more normal load transfer with the potential to minimize stress-shielding

The device profile is rectangular, with a hollow core to promote bone graft integration and fusion between the endplates.
The implant is available in various footprints and heights to accommodate patient variability and is manufactured from a titanium alloy per ASTM F3001.
Visual Density Markers
DHD - DEXA HIGH DENSITY
DMD - DEXA MEDIUM DENSITY
DLD - DEXA LOW DENSITY